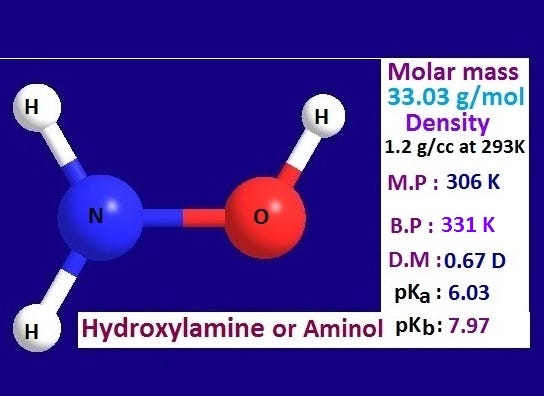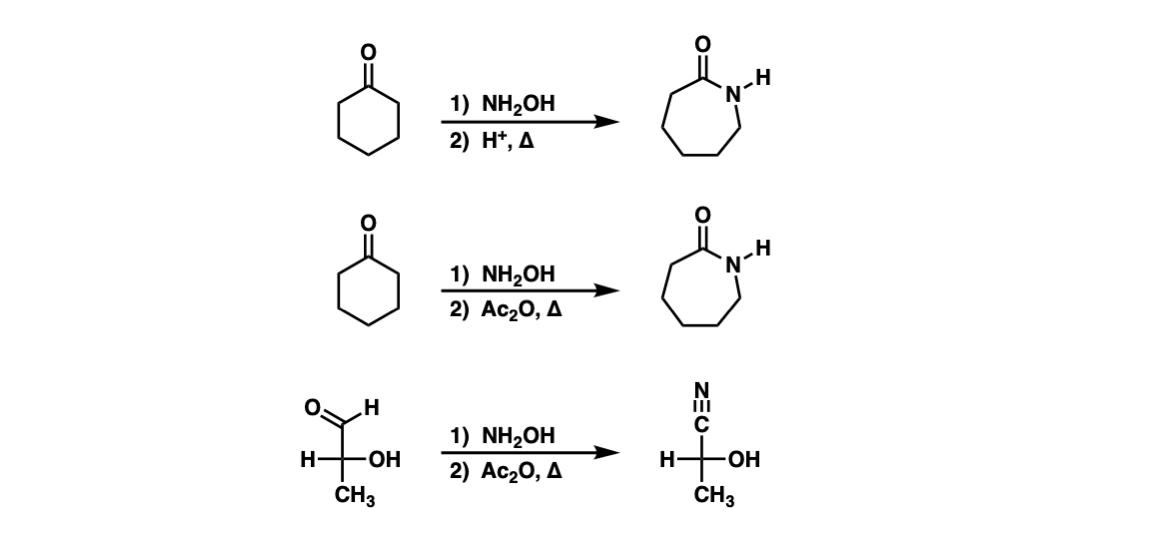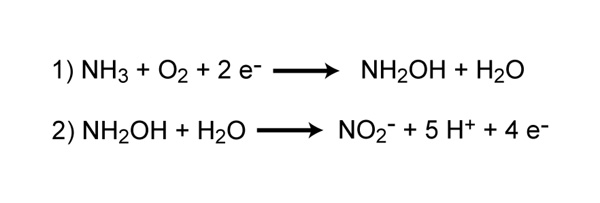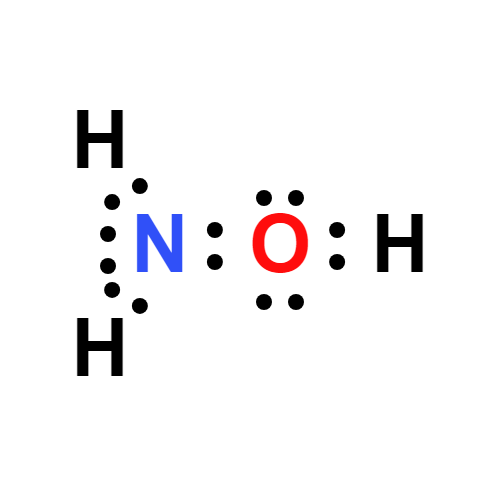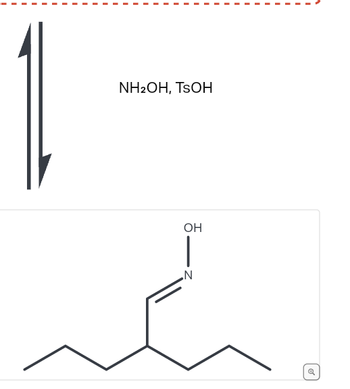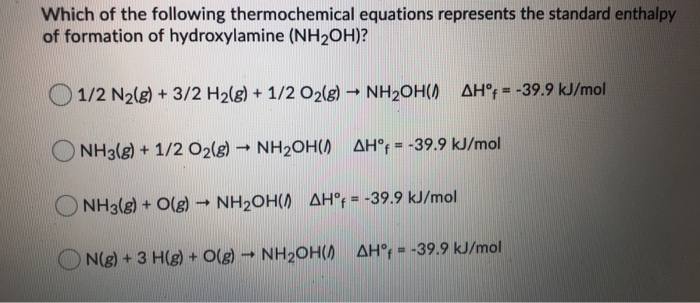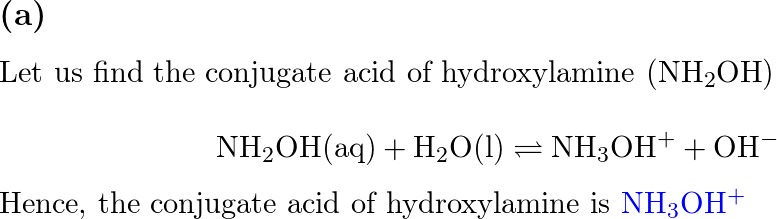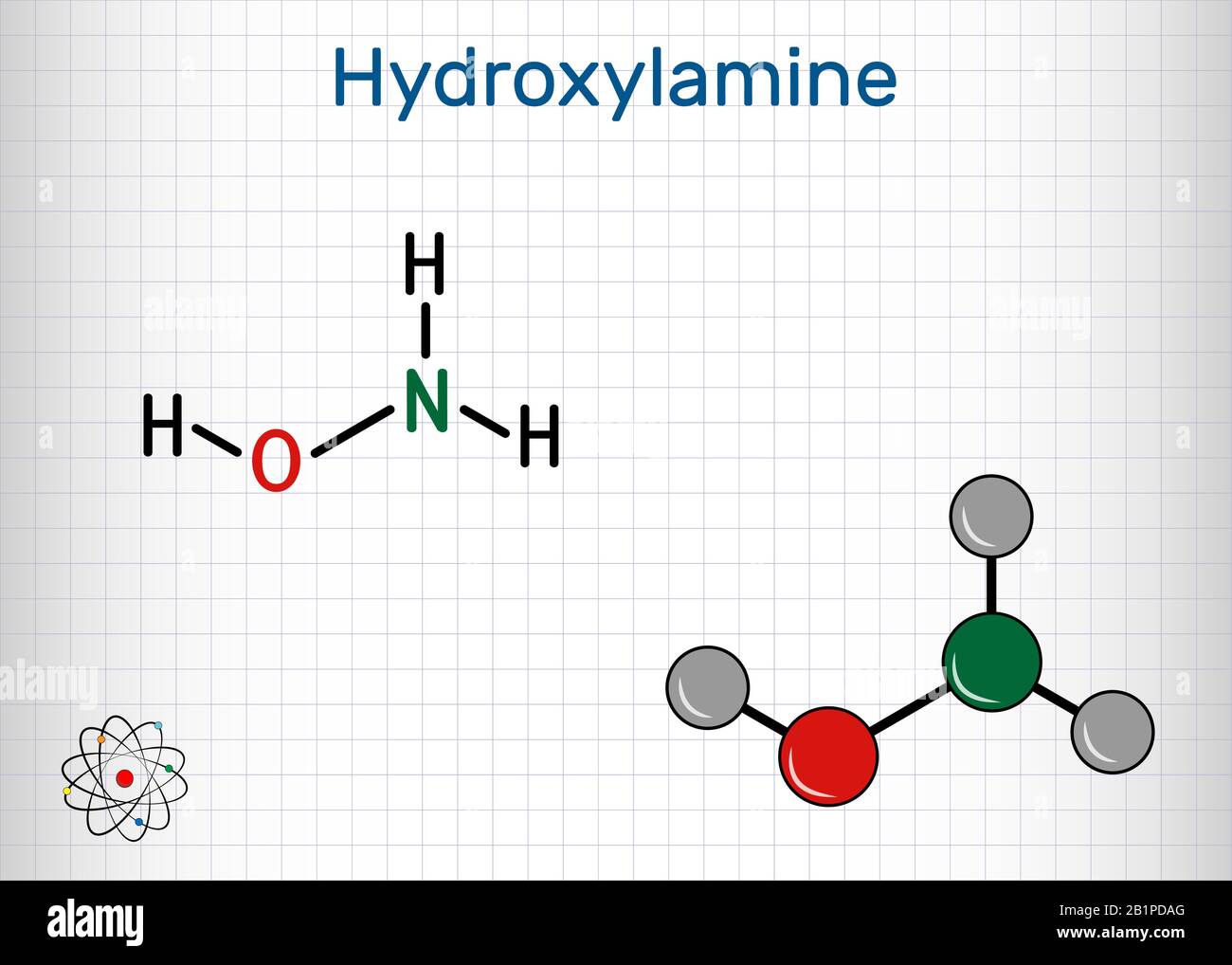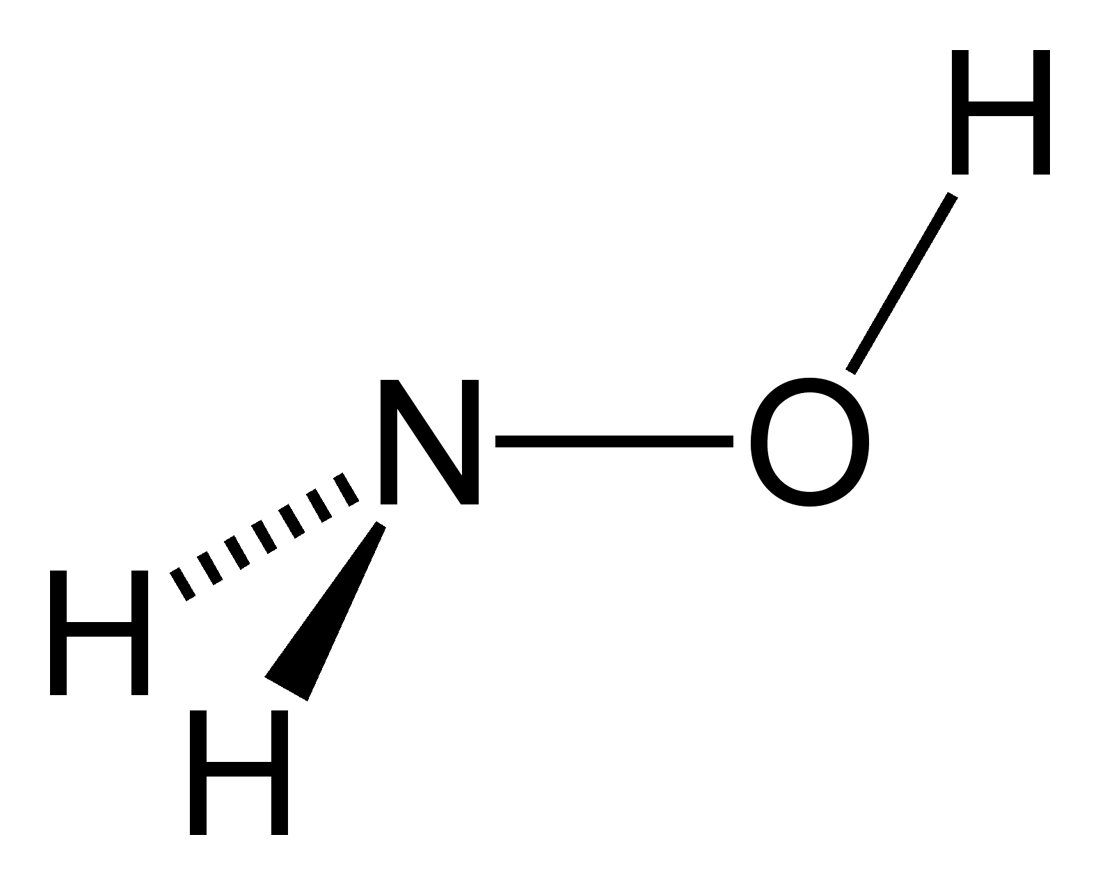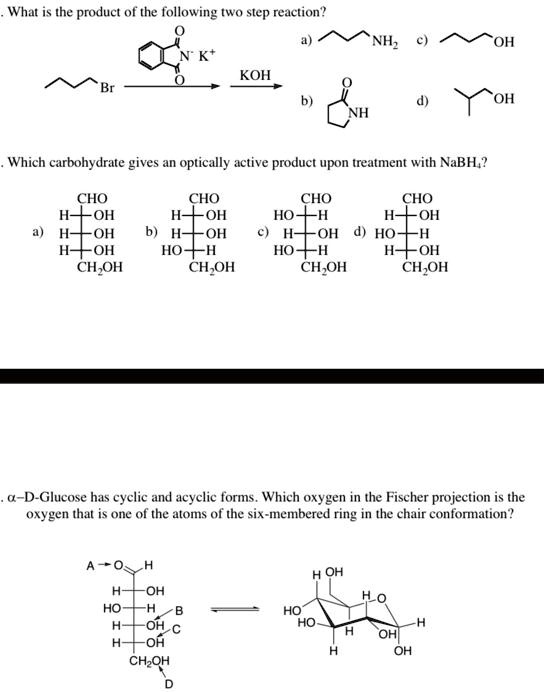
SOLVED: What is the product of the following two-step reaction? NH3 + KOH â†' NH2OH + H2O Which carbohydrate gives an optically active product upon treatment with NaBH4? CHO H-OH H-OH H-OH

Thermal Decomposition of NH2OH and Subsequent Reactions: Ab Initio Transition State Theory and Reflected Shock Tube Experiments | The Journal of Physical Chemistry A
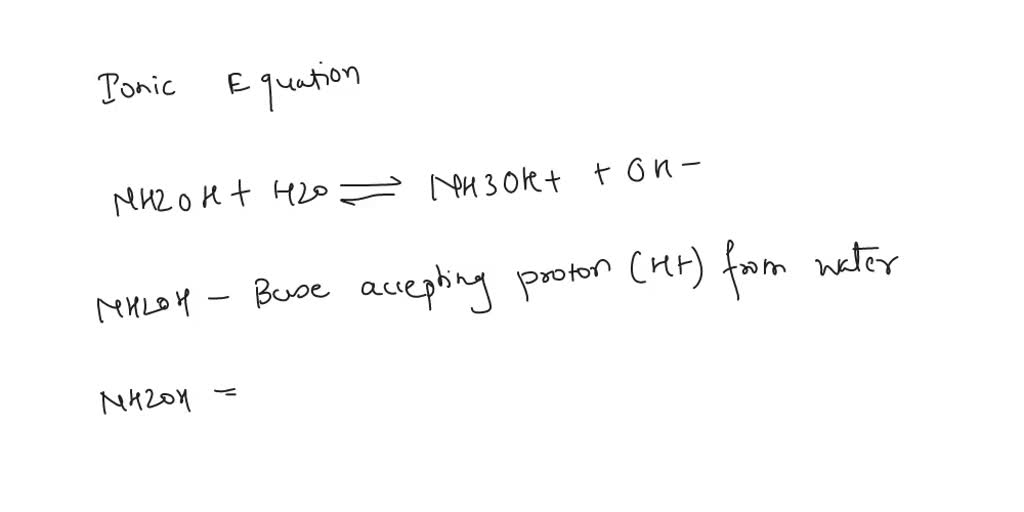
SOLVED: Write the net ionic equation to show that hydroxylamine (NH2OH) behaves as a Bronsted-Lowry base in water.
![SOLVED: 5. Hydroxylamine, NH2OH, reacts with water: NH2OH + H2O -> HNHOH* + OH- a) Is it acting as an acid or as a base? b) Identify its conjugate. c) The [OH-] SOLVED: 5. Hydroxylamine, NH2OH, reacts with water: NH2OH + H2O -> HNHOH* + OH- a) Is it acting as an acid or as a base? b) Identify its conjugate. c) The [OH-]](https://cdn.numerade.com/ask_images/db627e0c62e74e1a9a6bc5925a1a5d0a.jpg)
SOLVED: 5. Hydroxylamine, NH2OH, reacts with water: NH2OH + H2O -> HNHOH* + OH- a) Is it acting as an acid or as a base? b) Identify its conjugate. c) The [OH-]

Instanton rate constants for reaction ( 4 ) in Model A and Model B .... | Download Scientific Diagram

Further validation and application of the NH2OH and HNO products of N2 | Download Scientific Diagram

Calculate the pH of a 0.050 M solution of hydroxylamine, NH2OH. (Kb = 6.6 x 10-9) | Homework.Study.com