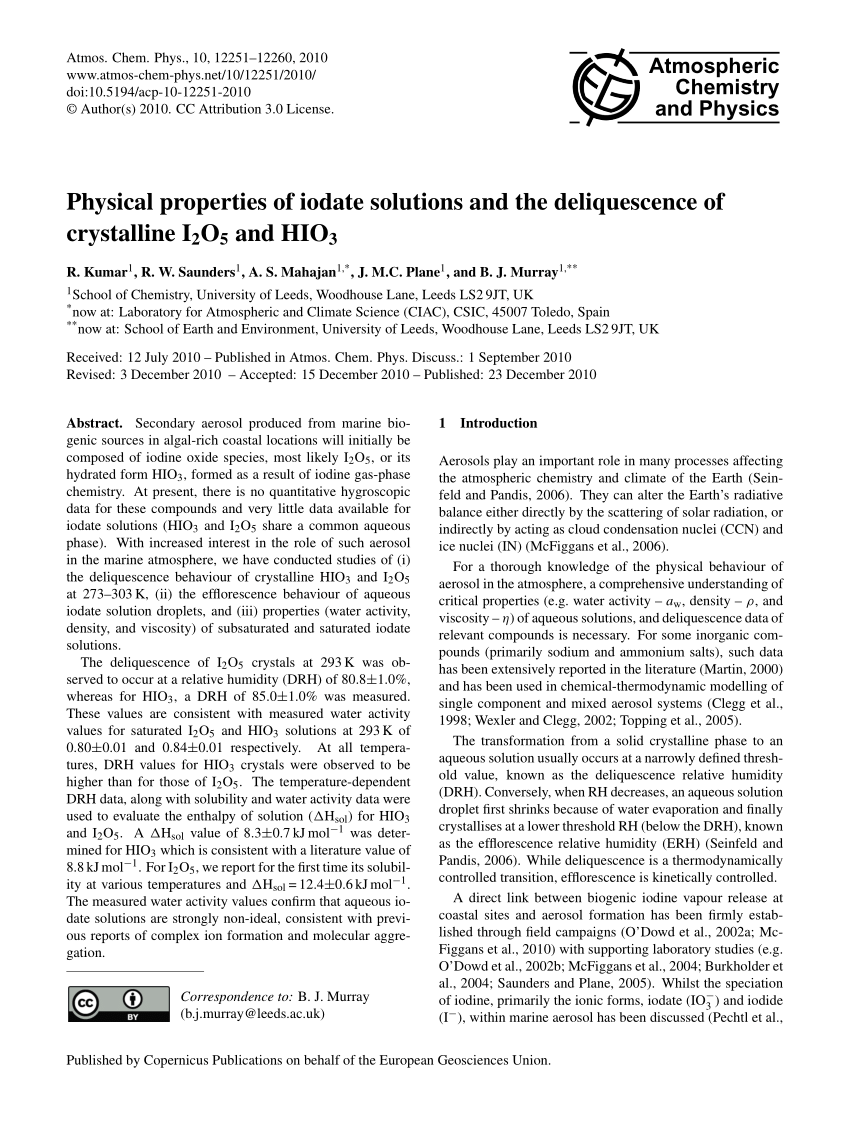
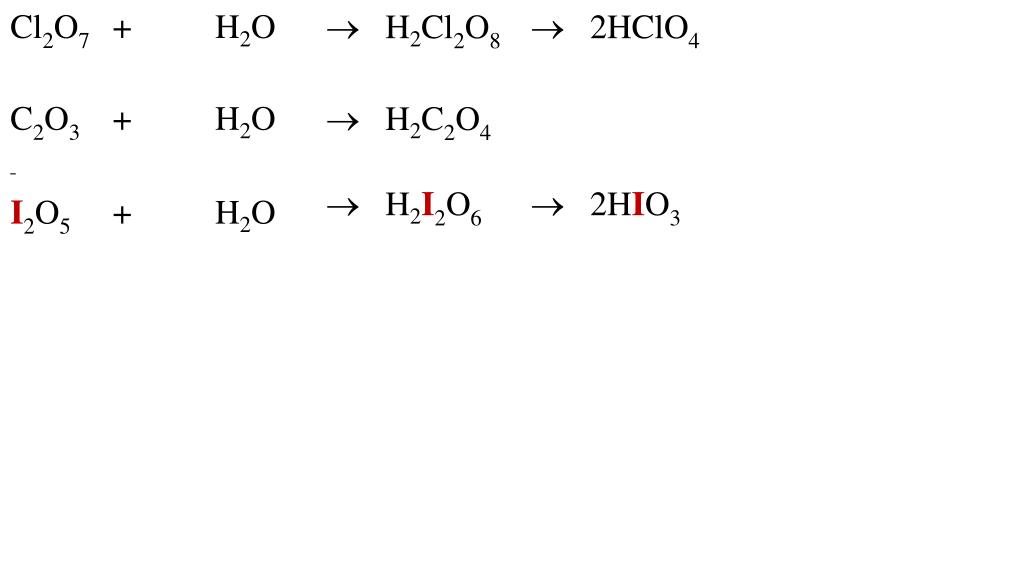SOLVED: In each of the following balanced oxidation-reduction equations, identify those elements that undergo changes in oxidation number and indicate the magnitude of the change in each case. I2O5 (g) + 5

Passivated Iodine Pentoxide Oxidizer for Potential Biocidal Nanoenergetic Applications | ACS Applied Materials & Interfaces
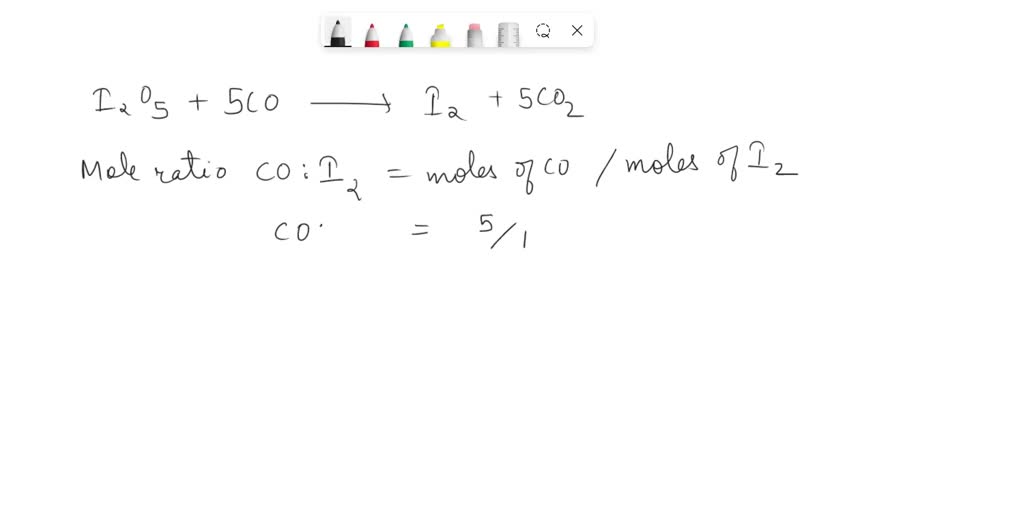
SOLVED: 1. I2O5 + 5CO —-> I2 + 5CO2 a) What is the mole to mole ratio of CO and I2? b) What is the mole to mole ratio of I2O5 and

SOLVED: Consider the reaction of 44.01 g of I2O5 and 101.0 g of BrF3 by the following reaction: (a) Balance the reaction first: I2O5(s) + BrF3(l) ==> IF5(l) + O2(g) + Br2(l) (
![I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry](https://pubs.acs.org/cms/10.1021/acs.joc.9b00765/asset/images/acs.joc.9b00765.social.jpeg_v03)
I2O5-Mediated Iodocyclization Cascade of N-(1-Arylallyl)pyridine-2-amines with Concomitant C═C Bond Cleavage: A Synthesis of 3-Iodoimidazo[1,2-a]pyridines | The Journal of Organic Chemistry

I2O5-Mediated 1,5-Cyclization of Aryldiynes with H2O: A Way To Access 3-Acyl-1-indenone Derivatives - ScienceDirect