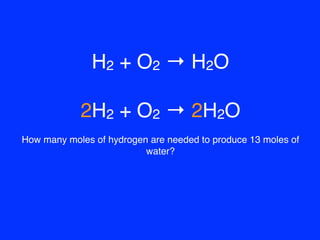
H2+O2=H2O balance the chemical equation @mydocumentary838. h2+o2=h2o balance the chemical equation. - YouTube
for the reaction H2 + 1/2 O2=H2O(l) △ Cp=7.63cal deg, △ H =68.3kcal ,what will be the value of △ H at 100degree celsius will b
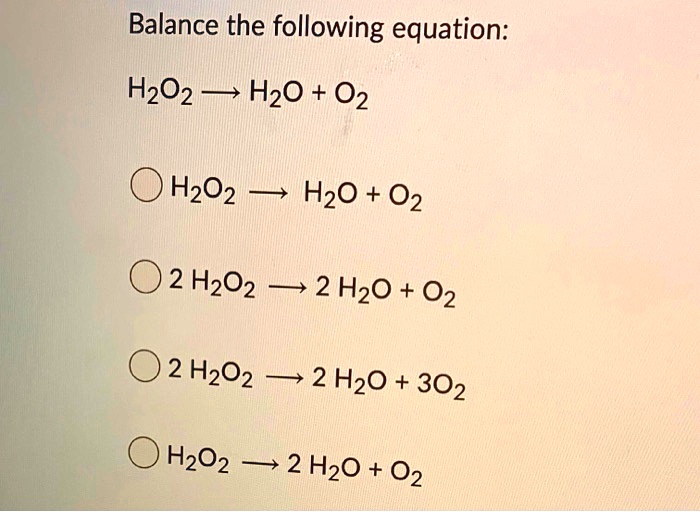
SOLVED: Balance the following equation: H2O2 H2O + O2 H2O2 H2O + O2 2 H2O2 2 H2O + O2 2 H2O2 2 H2O + 3 O2 H2O2 2 H2O + O2

Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

Balance the following chemical equation H2O2+O3⇒H2O+O2 Indicating the changes in oxidation numbers of oxygen, the equivalent weight of H2O2 this reaction.
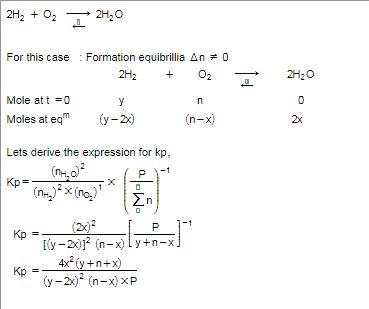
n mole each of h2o h2 o2 r tken in closed container at temperature t if y mole of h2 r disasssociated at equillibrium n equillibrium pressure is p the cgt66gee -Chemistry -

2H202 alkaline medium *2H20 + 02 the proposed mechanism is as given below : (1) H2O2 +1 → H2O+IO (slow) (2) H202 + 10 + H20+1+02 (fast) (i) Write rate law the

![PDF] Initiation in H2/O2: Rate constants for H2+O2→H+HO2 at high temperature | Semantic Scholar PDF] Initiation in H2/O2: Rate constants for H2+O2→H+HO2 at high temperature | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/59651c1a79d5be73d45e3b492f6f4396965dd05f/5-Table1-1.png)