
CO-ADSORPTION OF CO, H2O AND MECHANISM OF WATER GAS SHIFT REACTION ON ZnO CATALYST SURFACE: A DENSITY FUNCTIONAL THEORY STUDY | Semantic Scholar

For the equilibrium CO + H2O CO2 + H2 .The relation between Kp and Kc at 25^o C and at 100^o C are: - Brainly.in
Kc for CO(g) +H2O(g) ⇌ CO2(g) +H2(g) at 986°C is 0.63. A mixture of 1 mole H2O(g) - Sarthaks eConnect | Largest Online Education Community

Reactions of Photoionization-Induced CO–H2O Cluster: Direct Ab Initio Molecular Dynamics Study | ACS Omega

Radiation-induced synthesis of formic acid in the H2O–CO system: A matrix isolation study - ScienceDirect
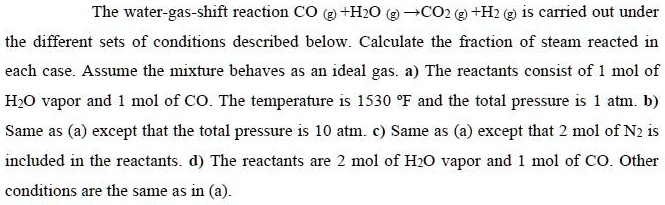
SOLVED: The water-gas-shift reaction CO (g) + H2O (g) â†' CO2 (g) + H2 (g) is carried out under different sets of conditions described below. Calculate the fraction of steam reacted in

64 The equilibrium constant the reaction, CO(g) + H2O (9) CO2 (g) + H2 (g) a certain temperature is 2.2. Initially one mole of CO and one mole of H2O are placed

Reaction of CO, H2O, H2 and CO2 on the clean as well as O, OH and H precovered Fe(100) and Fe(111) surfaces - Catalysis Science & Technology (RSC Publishing)
An equilibrium mixture, CO(g) + H2O(g) ⇋ CO2 (g) + H2 (g), present in a vessel of one litre capacity at 1000 K - Sarthaks eConnect | Largest Online Education Community

CO+H2O=CO2+H2 balance the chemical equation by law of conservation of mass @mydocumentary838. - YouTube

I 6.4 The equilibrium constant the reaction, CO + H20 (g) CO2 (g) + H2 (9) a certain temperature is 2.2. Initially one mole of CO and one mole of H2O are


![co(h2o)6]3+ is inner orbital octahedral but[fe(h2o) 6]2+ is outer orbital so why co(h2o)6]3+ is inner orbital octahedral but[fe(h2o) 6]2+ is outer orbital so why](https://cdn.eduncle.com/library/scoop-files/2021/9/can_image_1631894248696.jpg)


![co(h2o)6]3+ is inner orbital octahedral but[fe(h2o) 6]2+ is outer orbital so why co(h2o)6]3+ is inner orbital octahedral but[fe(h2o) 6]2+ is outer orbital so why](https://cdn.eduncle.com/library/scoop-files/2021/9/can_image_1631894219872.jpg)



![Co(H2O)6]2+ Co(H2O)6]2+](https://tp-inorga-1-13.webself.net/file/si532904/Spectre%20Co6-fi8187013x610.png)