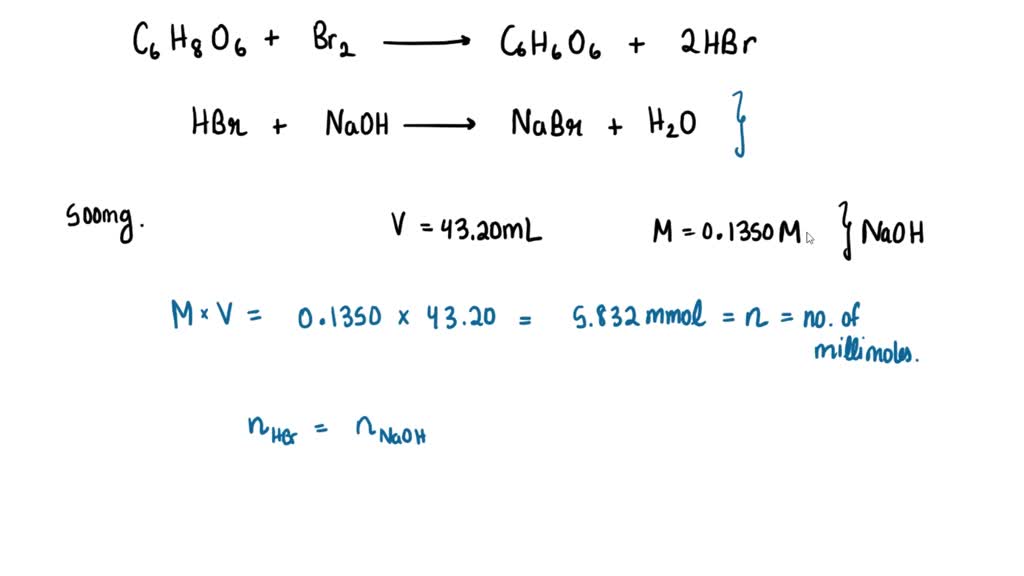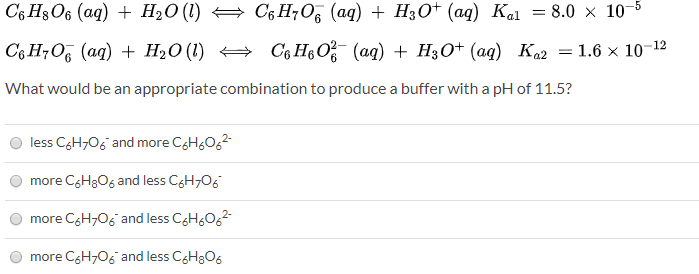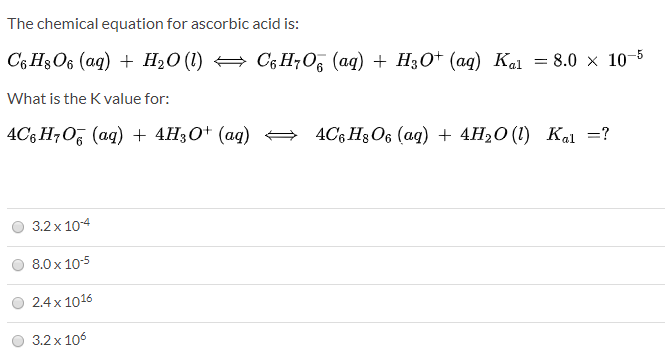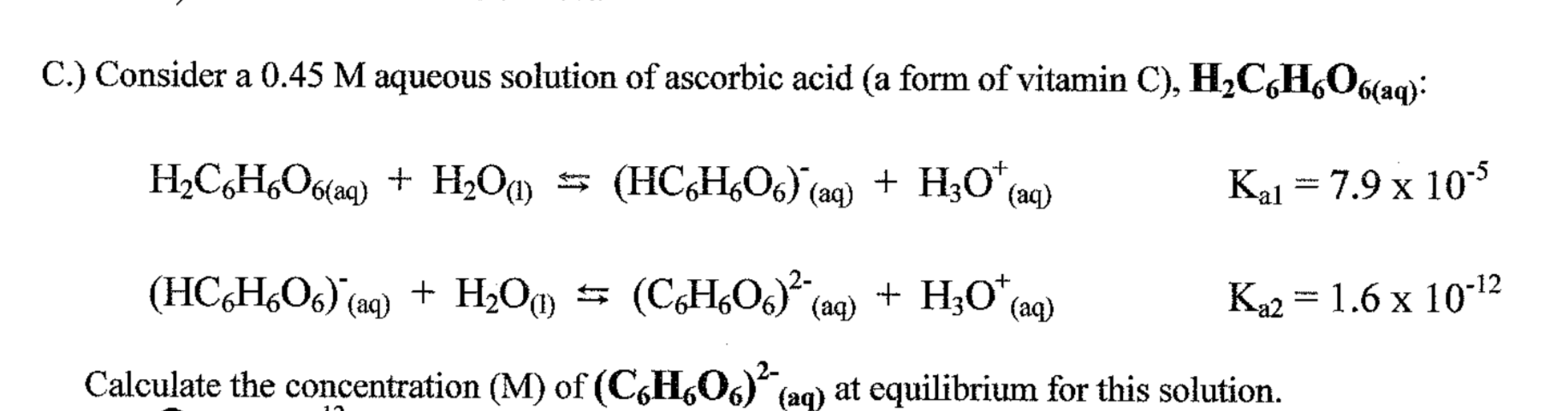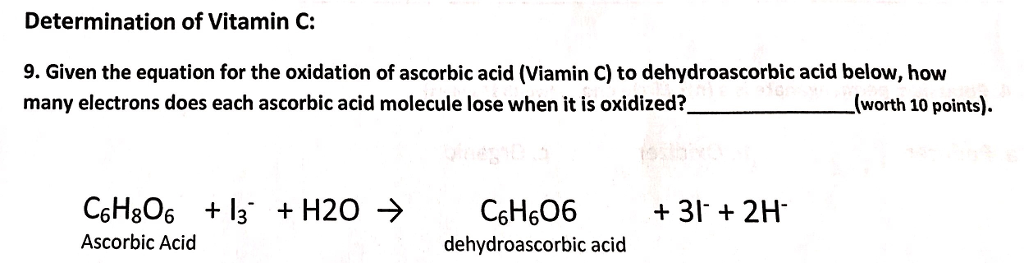How the Multiple Antioxidant Properties of Ascorbic Acid Affect Lipid Oxidation in Oil-in-Water Emulsions | Journal of Agricultural and Food Chemistry
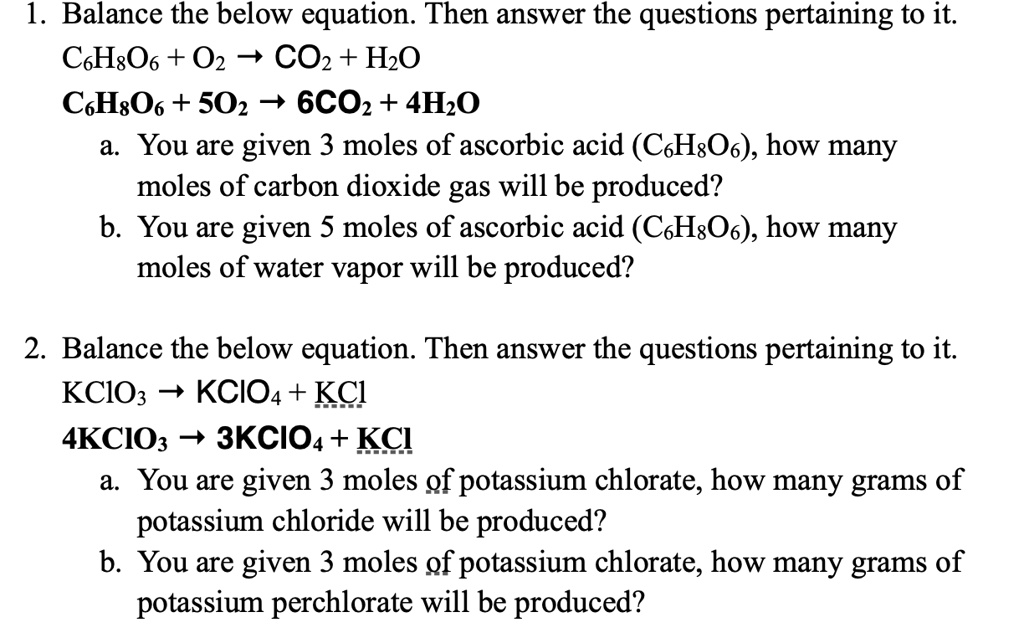
SOLVED: Balance the below equation. Then answer the questions pertaining to it. C6H8O6 + O2 -> 6CO2 + 4H2O CH2O + 5O2 -> 6CO2 + 4H2O a. You are given 3 moles
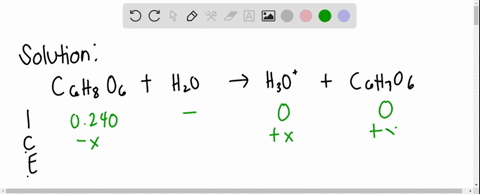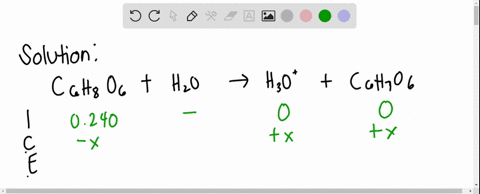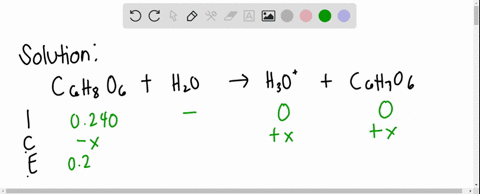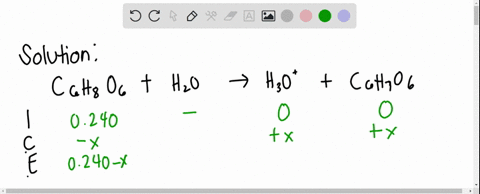
SOLVED: Ascorbic acid (vitamin C, C6H8O6) is a diprotic acid (Ka1 = 8.0×10–5 and Ka2 = 1.6×10–12). What is the pH of a 0.240 M solution of ascorbic acid?
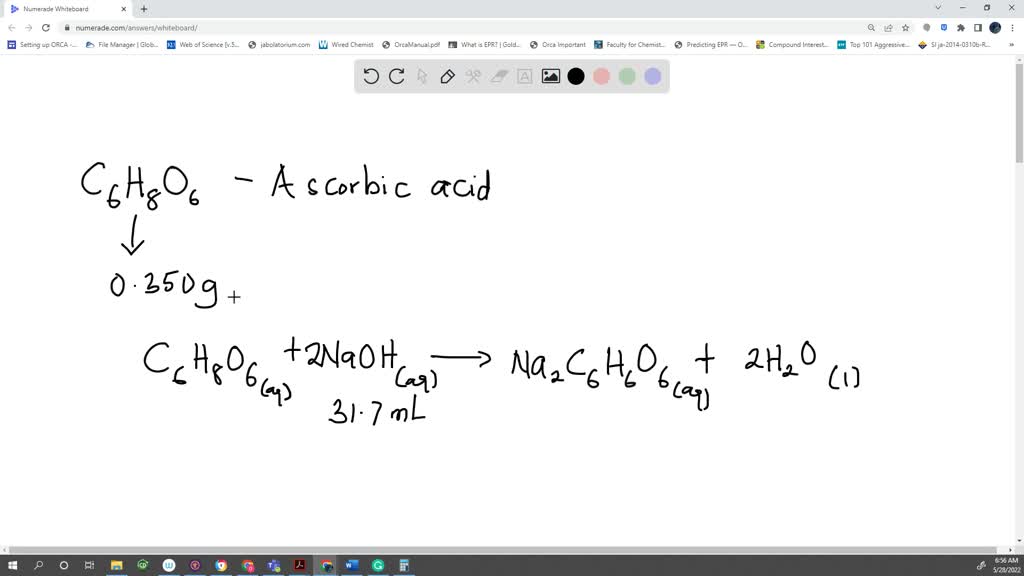
SOLVED: A sample of ascorbic acid, with the formula C6H8O6, is to be studied by titration. First, 0.350 grams of the acid are added to a flask. Sodium hydroxide solution is then
![SOLVED: Ascorbic acid (C6H8O6) is a diprotic acid. Its Ka values are listed below. What is the [C6H8O6-2] in a 0.15 M solution of ascorbic acid? C6H8O6 (aq) + H2O (l) ⇌ SOLVED: Ascorbic acid (C6H8O6) is a diprotic acid. Its Ka values are listed below. What is the [C6H8O6-2] in a 0.15 M solution of ascorbic acid? C6H8O6 (aq) + H2O (l) ⇌](https://cdn.numerade.com/ask_images/55e213a8c21947539aafd32122cc8bef.jpg)
SOLVED: Ascorbic acid (C6H8O6) is a diprotic acid. Its Ka values are listed below. What is the [C6H8O6-2] in a 0.15 M solution of ascorbic acid? C6H8O6 (aq) + H2O (l) ⇌

NCERT Solution Intext 2.11: Calculate the mass of ascorbic acid (Vitamin C, C6H8O6) to be dissolve - YouTube

Schema 2. Reaction of AgNPs with polysaccharide (Cx(H2O)y) and ascorbic... | Download Scientific Diagram

Solved: Chapter 8 Problem 8P Solution | ConnectPlus Chemistry Access Card For General, Organic & Biological Chemistry 1st Edition | Chegg.com