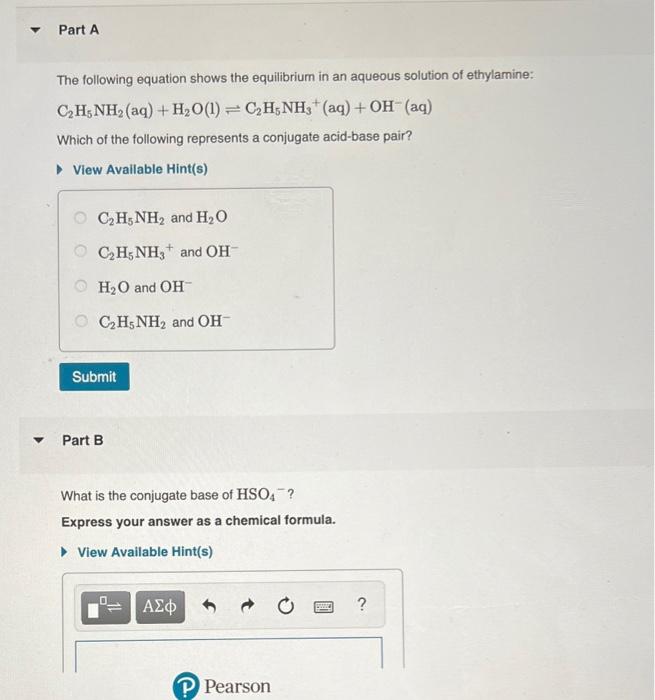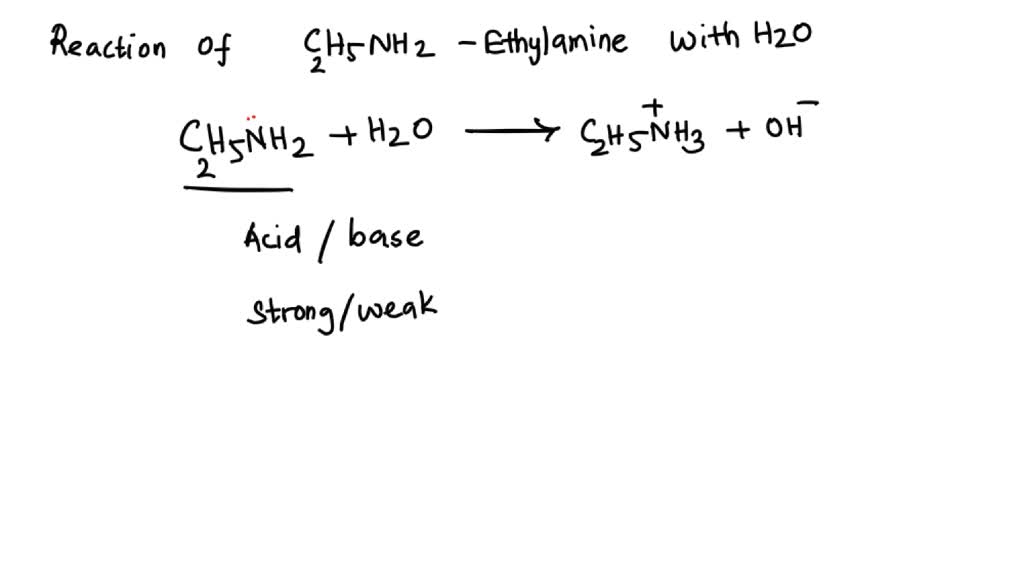
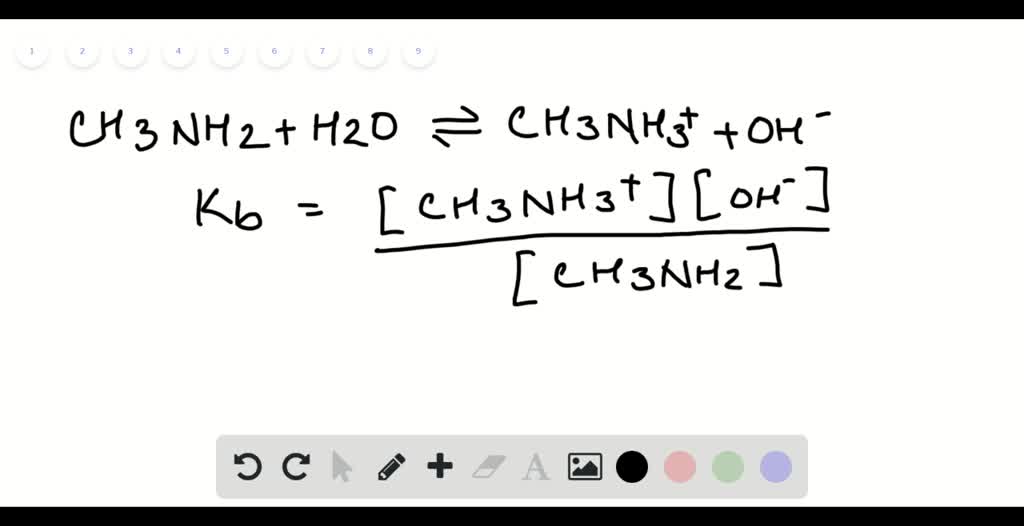
SOLVED: 5. When added to water, ethylamine undergoes the following reaction: C2H5NH2 + H2O C2H5NH3+ + OH- Is ethylamine an acid or a base in this reaction? Strong or weak? How do
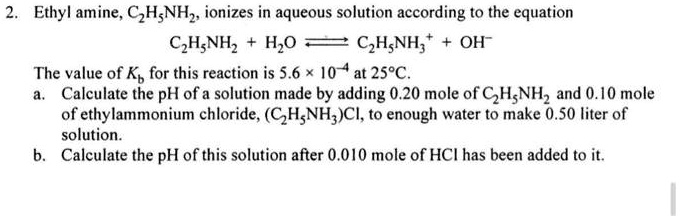
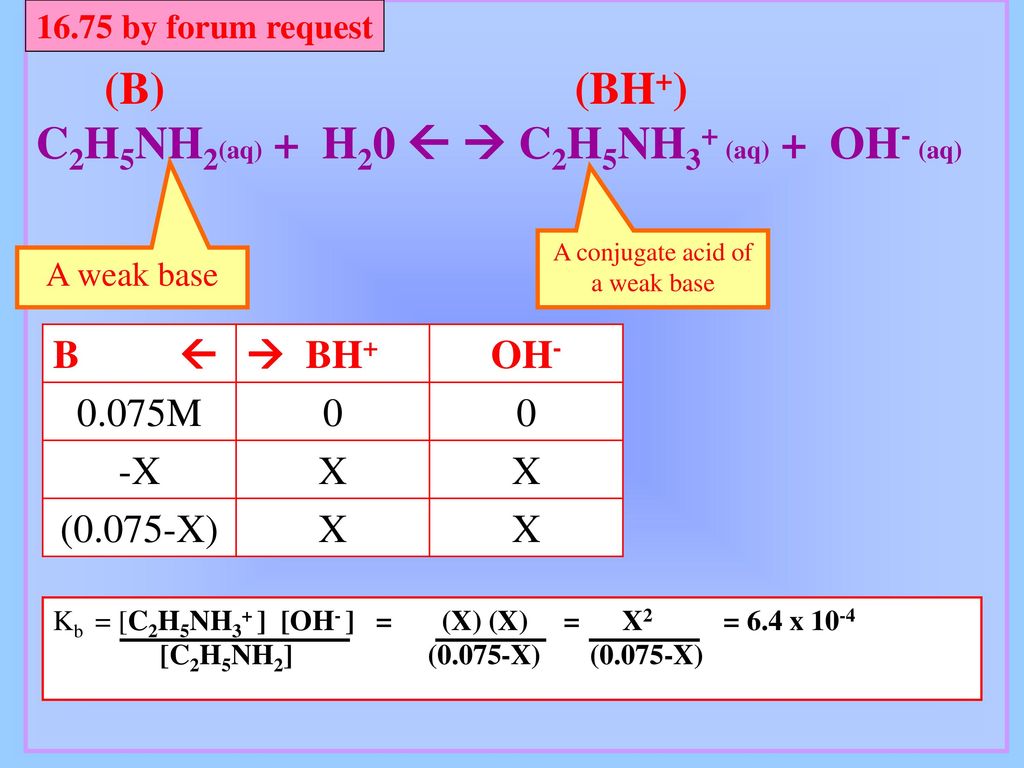
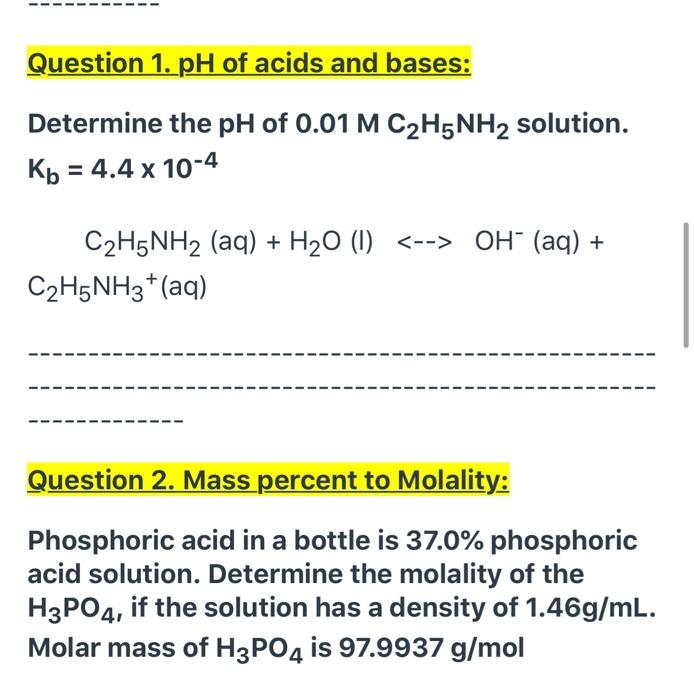
SOLVED: Ethylamine, C2H5NH2, ionizes in aqueous solution according to the equation C2H5NH2 + H2O ⇌ C2H5NH3+ + OH-. The value of Kb for this reaction is 5.6 x 10^4 at 25°C. Calculate
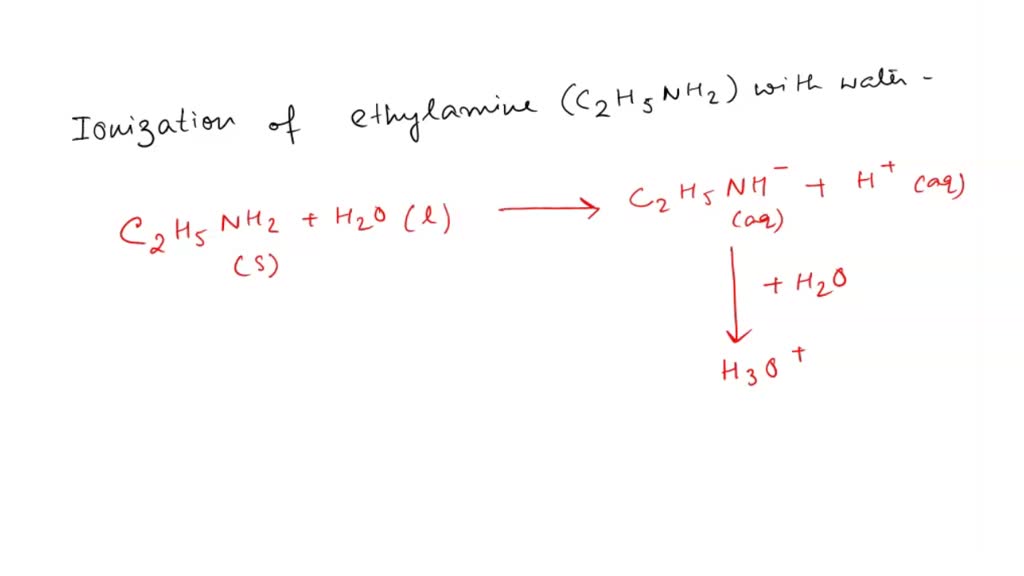
SOLVED: Write the equation for the ionization of ethylamine (C2H5NH2), a weak molecular base, with water

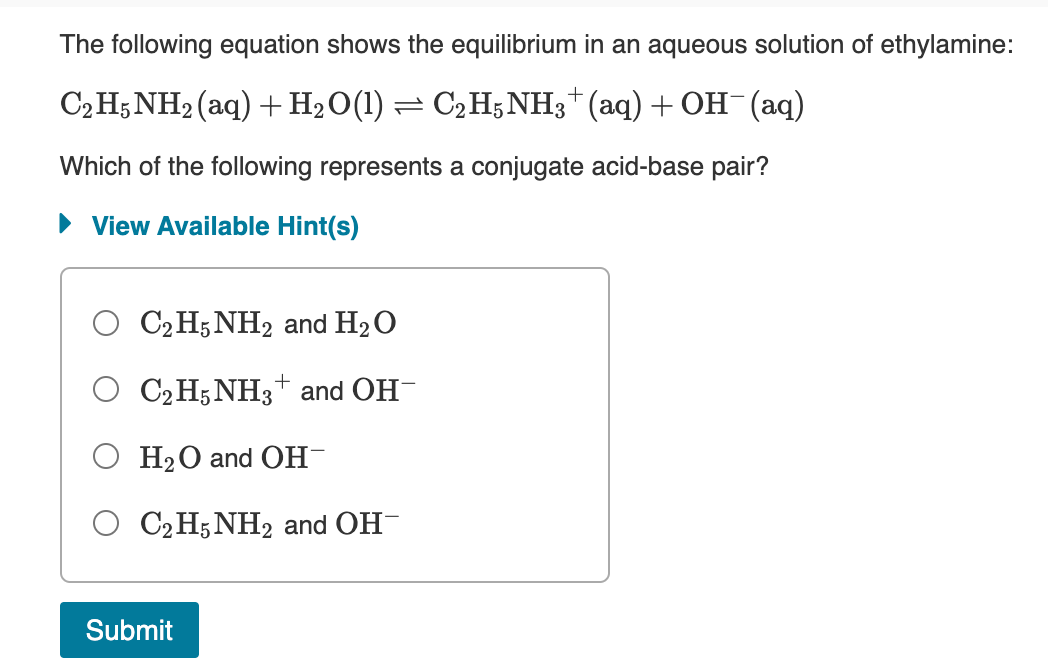
SOLVED: In the following chemical equation, identify the conjugate acid-base pairs: C2H5NH2 + H2O = C2H6NH+ + OH- C2H5NH2 (base), C2H6NH+ (acid); H2O (acid), OH- (base)
![SOLVED: The base-dissociation constant of ethylamine (C2H5NH2) is 6.4 × 10-4 at 25.0 °C. The [H+] in a 1.6 × 10-2 M solution of ethylamine is M. SOLVED: The base-dissociation constant of ethylamine (C2H5NH2) is 6.4 × 10-4 at 25.0 °C. The [H+] in a 1.6 × 10-2 M solution of ethylamine is M.](https://cdn.numerade.com/project-universal/previews/776905ac-1583-4182-adc4-a5a01e13e224.gif)
SOLVED: The base-dissociation constant of ethylamine (C2H5NH2) is 6.4 × 10-4 at 25.0 °C. The [H+] in a 1.6 × 10-2 M solution of ethylamine is M.

Max. Marks : 70 an 4. Complete the following reaction. CO → A -KI NH KOH (alc.) CHI NH H2O CO - HO C + C2H5NH2 Ethylamine
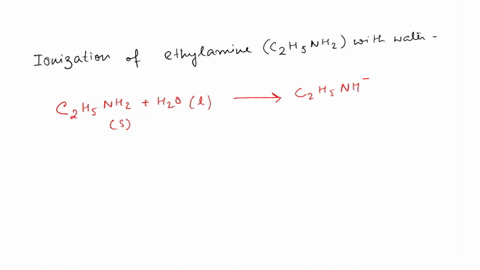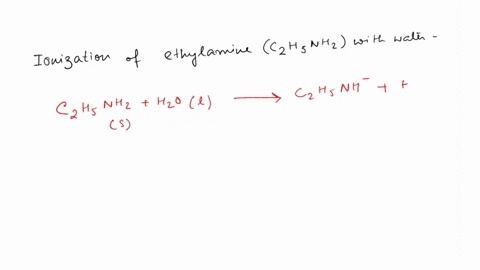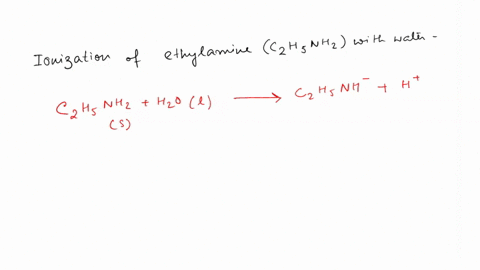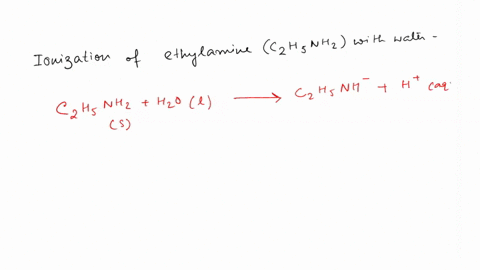




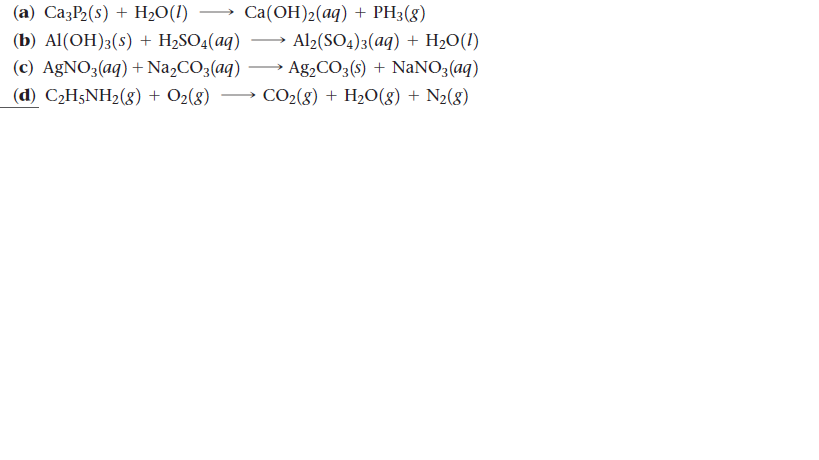
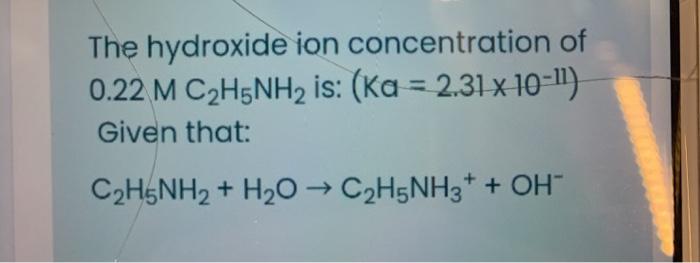



![Exercice [CHIMIE_Tle C,D,E, : La classification des couples acide/base dans l'eau] Exercice [CHIMIE_Tle C,D,E, : La classification des couples acide/base dans l'eau]](https://fasoeducation.bf/cours_esu/secondaire/terminale/sp/chimie/classification_couples_acide_base_eau/res/image_group.png)