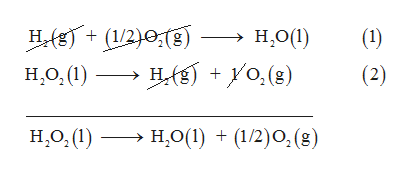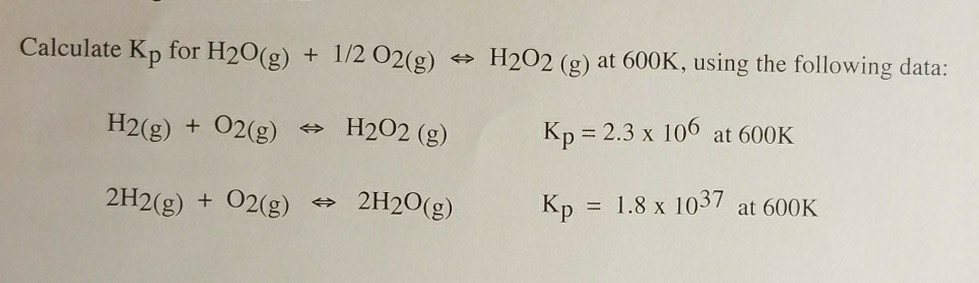4 (i) H2O2 + 03--> H2O + 202 (ii) H202+ Ag20 >2Ag + H2O+O2 Role of hydrogen peroxide in the above reaction is respectively: (1) Oxidizing in (i) and reducing in (i) (
1) H2O2 + O3 → H2O +2O2 2)H2O2 +Ag2O →2Ag +H2O +O2 Determine whether H2O2 is oxidised or reduced in the above reaction? Explain.

Comment équilibrer : H2O2 → O2 + H2O (peroxyde d'hydrogène, dioxygène, eau) | Physique-Chimie - YouTube

16.38d | Would hydrogen peroxide be a suitable candidate for fuels: H2O2(l) → H2O(g) + 1/2O2(g) - YouTube

Determine enthalpy of formation H2O2(1), using listed enthalpies of reaction: N2H4(1) + 2H2O2(1) → N2(g) + 4H2O(l); AH; =-818 kJ/mol N2H4(1) + O2(g) → N2(g) + 2H2O(1); 4.H; = -622 kJ/mol H2(g) +

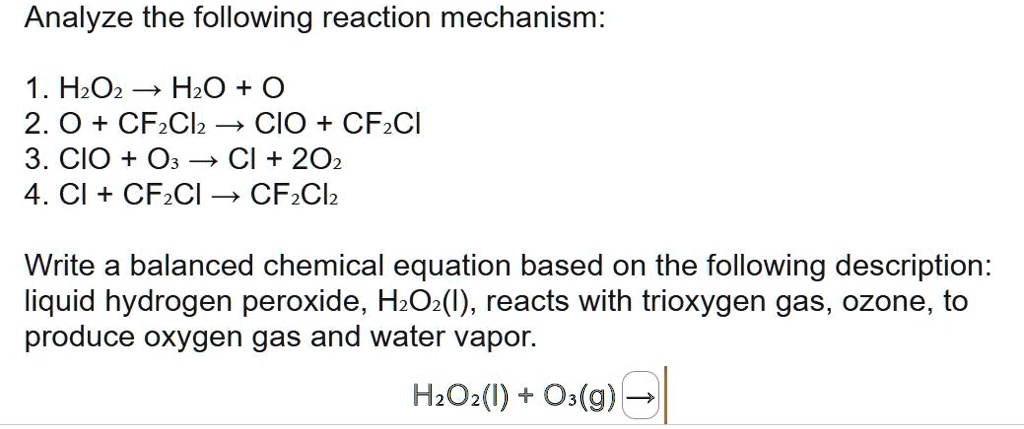
SOLVED: Consider the following chemical reaction mechanism: 1. H2O2 –> H2O + O (slow) 2. O + CF2Cl2. –> ClO + CF2Cl 3. ClO +O3 –> Cl + 2O2 4. Cl +
✓ Solved: Calculate ΔG^∘ for H2 O(g)+1 / 2 O2(g) ⇌ H2 O2(g) at 600.K using the following data: H2(g)+O2(g)...

SOLVED: Calculate ΔG° for the reaction H2O(g) + 1/2O2(g) = H2O2(g) at 603 K using the following data: H2(g) + O2(g) = H2O2(g) K = 1.21037 at 603 K 2H2(g) + O2(g) =
Why is the answer B? Can someone explain this to me and why other options are incorrect. I assumed that H2O2 will decompose rapidly to form H20 and O2 with MnO2 as
I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community