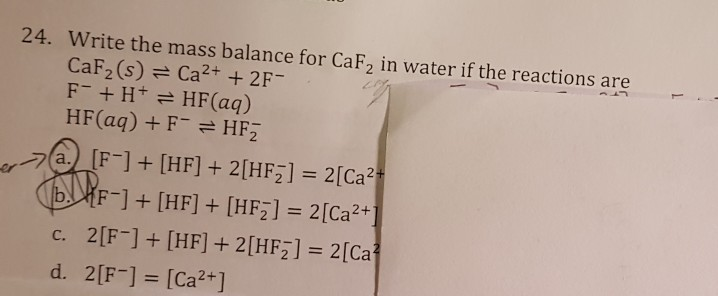Optical Coating Material Calcium Fluoride CaF2 Granule Customized Size China - China Coating Material, Calcium Fluoride | Made-in-China.com

SOLVED: Consider the unbalanced chemical equation below: CaSiO3(s) + HF(g) â†' CaF2(aq) + SiF4(g) + H2O(l) Suppose a 34.4 g sample of CaSiO3 is reacted with 31.6 L of HF at 27.0

CaF2 97 %. Le spath fluor Poudre humide/ CaF2 en poudre, de spath fluor Poudre humide - Chine Fluoraper, le fluorure de calcium

Solubility Data for the CaF 2 + MnSO 4 + H 2 O Ternary System at 298.15... | Download Scientific Diagram
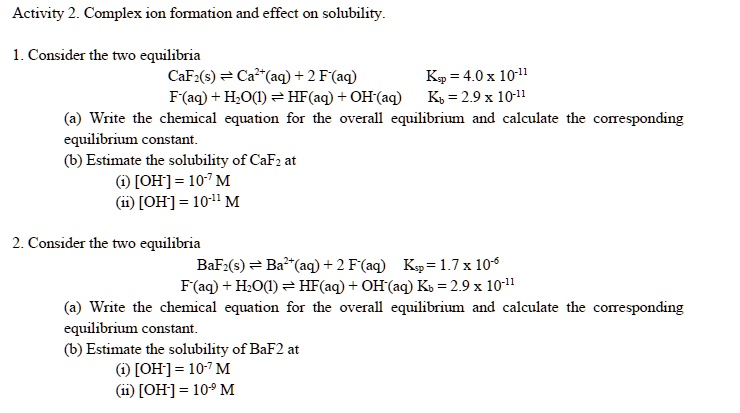

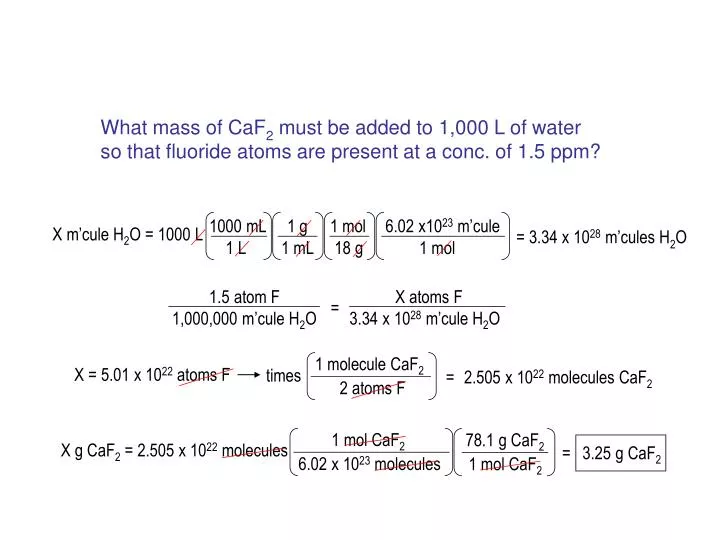






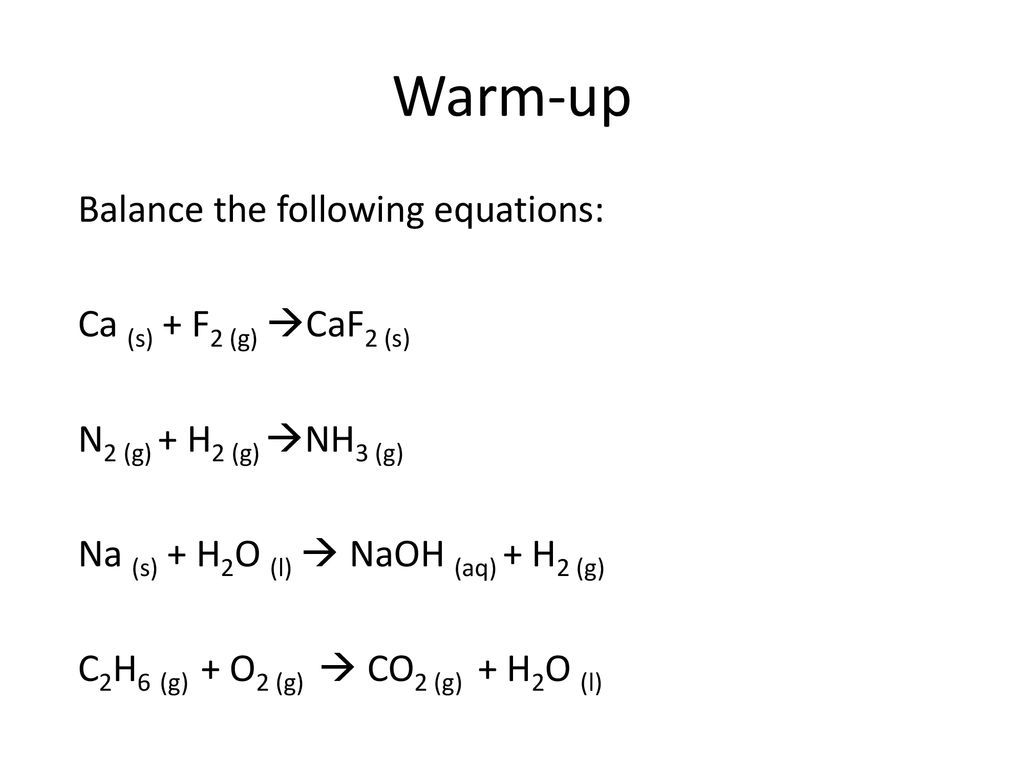

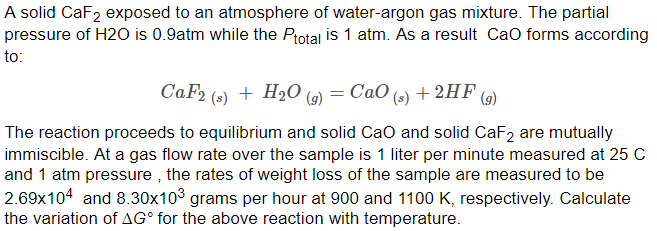






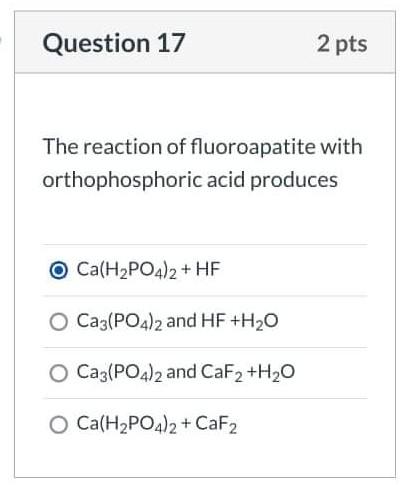
![Solved [2] (a) Consider the reaction: | Chegg.com Solved [2] (a) Consider the reaction: | Chegg.com](https://media.cheggcdn.com/study/0cd/0cd25e73-2e52-4764-8524-5f8d96b0fc58/image)