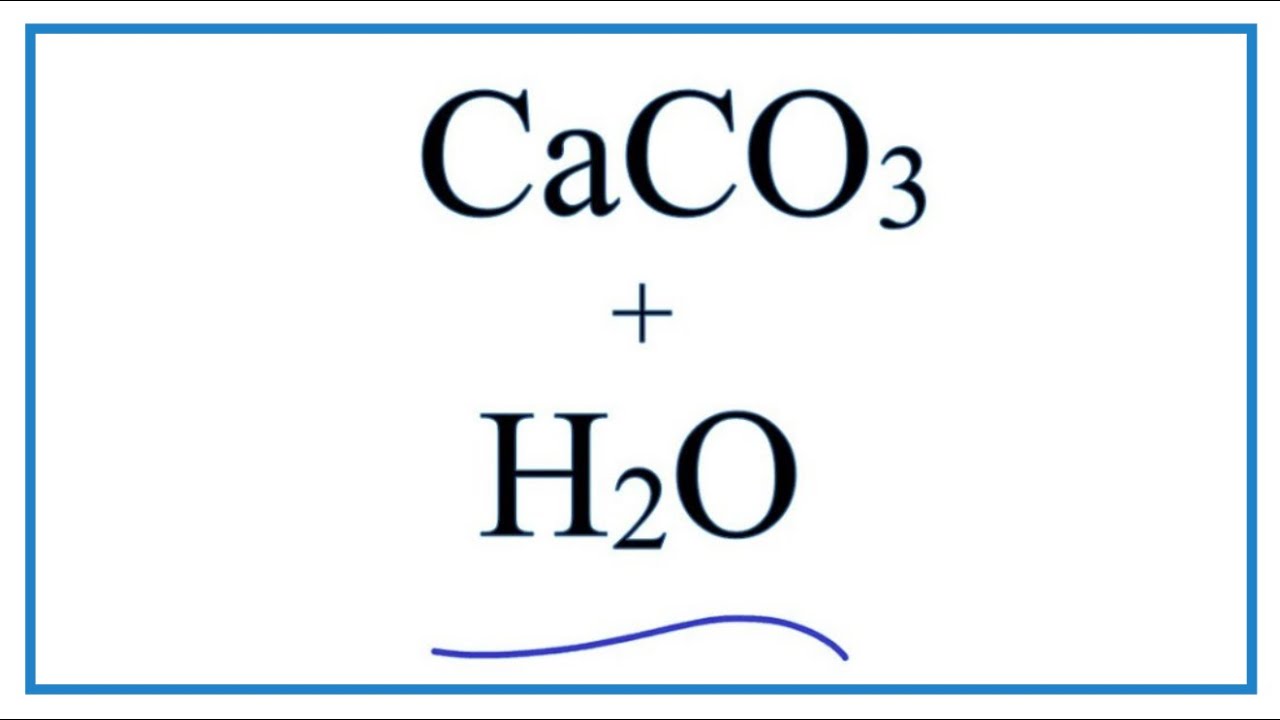
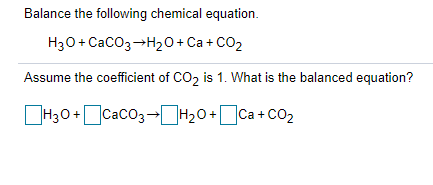Section - B 15. Complete and balance the following chemical equations : (1) NaOH(aq) + Zne) → (ii) CaCO3(e) + H2O + CO2(2)→ (iii) HCl(aq) + H20) ▻ OR

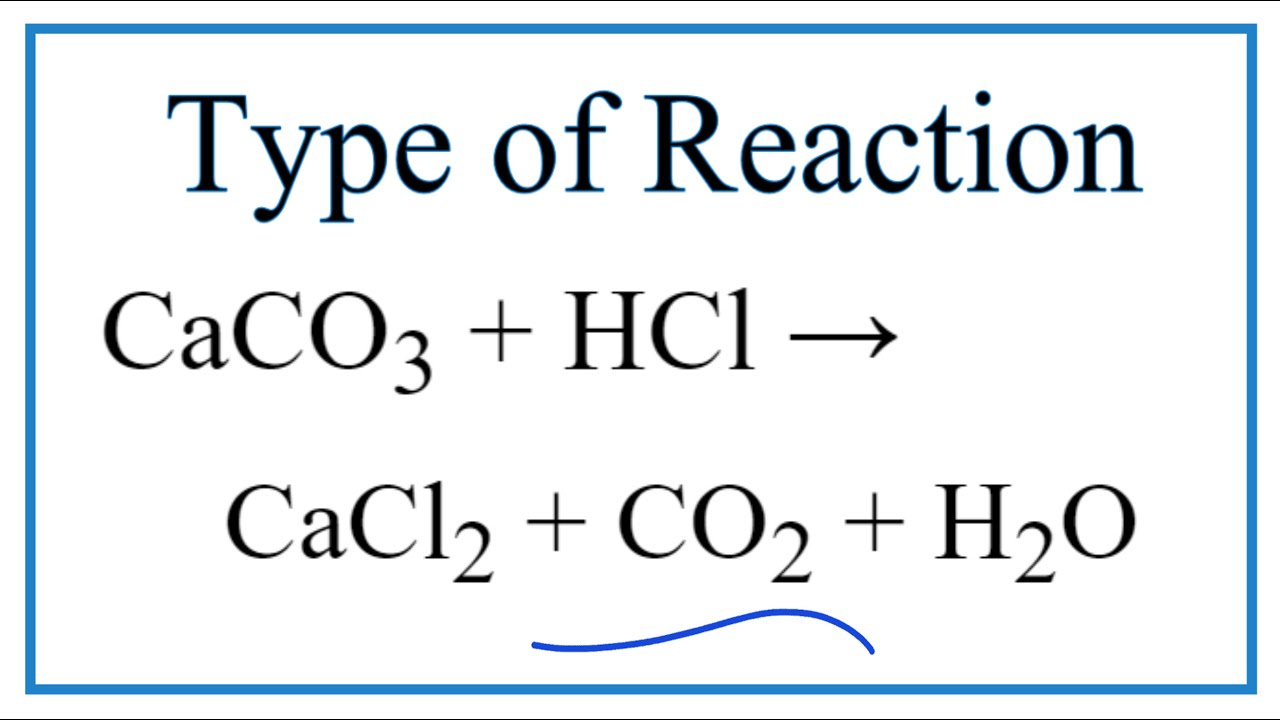
HCl+CaCO3=CaCl2+H2O+CO2 balance the chemical equation @mydocumentary838. hcl+caco3=cacl2+h2o+co2 - YouTube
The mass of CaCO3 required to react completely with 20 mL of 1.0 M HCL as per the reaction CaCO3+2HCl–>CaCl2+CO2+H2O
40. Consider the reaction CaCO3+2HCL (l) 》CaCl2+CO2+H2O (l).what mass of CaCO3 is required to react with 20mL 1M HCL?

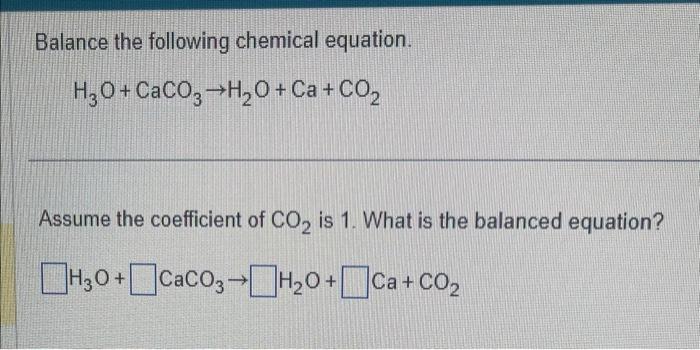
Q8. Balance the following reactions: a) CaCO3 + HCl +CaCl2 + H2O + CO2 b) H2O + H2 + O2 c) N2 + H2 → NH d) NaOH + H2SO4 → Na

SOLVED: CaCO3(s) - CaO(s) + CO2(g) ΔH = 175 kJ Ca(OH)2(s) - H2O(l) + CaO(s) ΔH = 67 kJ Ca(OH)2(s) + 2 HCl(g) â†' CaCl2(s) + 2 H2O(l) ΔH = -198 kJ

How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O | How to Balance CaCO3 + HCl = CaCl2 + CO2 + H2O Hey there! Are you struggling with balancing
16. In a chemical reaction, caco3+2hcl= cacl2 +co2+h2o. 25ml hcl and 0.75M Calculate the amount of caco3